Pfzier & Bextra
What Happened
Pfizer launched Bextra, a drug apart of the revolutionary class of painkillers known as Cox-2 inhibitors. Pfizer and its marketing partner, Pharmacia, planned to sell Bextra as a treatment for acute pain, so long as the FDA permitted it.
The FDA found that the drug was not safe for patients with high risk of stroke or heart attacks. They went on to only approve Bextra for arthritis and menstrual cramps.
Pfizer promoted the drug for it’s “off-label” uses, which is highly illegal. By the time Bextra was taken off the market four years later, more than half of its $1.7 billion in profits had come from prescriptions written for uses that the FDA had rejected.
Signs Missed
Internal Pfizer documents show that Pfizer and Pharmacia used a multimillion dollar medical education budget to pay hundreds of doctors as speakers and consultants to promote Bextra for the aforementioned off-label uses. Further, an internal marketing plan called for training physicians “to serve as public relations spokespeople.”
Estimated Damages:
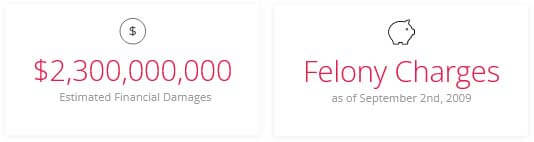
All information in this case study is based on data that was found on public domain and official public records.